Host-microbiota Interaction
Our research is focused on understanding how specific members of the gut microbiota impact the host immune system in both healthy and diseased states. In particular, our lab focuses on the characterization of the unique life-cycle of the gut commensal Segmented Filamentous Bacteria, its cross-talk with the host, and its immunostimulatory potential to promote both broad and targeted pathogen resistance.
The gut microbiota plays an integral part in shaping the host immune system and amongst its many functions, helps to protect the host from pathogens. How individual bacterial species contribute to colonization resistance is still poorly understood.
Segmented Filamentous Bacteria (SFB) is a common commensal in many vertebrates and has unique immunostimulatory. In mice, SFB triggers controlled physiological inflammation early in life, leading to the induction of Th17 cells, IgA responses, and enhanced immune reactivity. SFB primes the host's immune system to promote resistance to a wide range of pathogens, inside and outside the gut, and is a potent mediator of the weaning reaction that prevents pathological imprinting of the immune system.
However, this heightened immune response can either worsen or alleviate disease severity in a growing list of murine disease models. The presence of SFB in the gut thereby acts as a biomarker for overall immune reactivity, pathogen resistance, and disease susceptibility.
SFB's strong immunomodulatory effects are linked to its close attachment to the small intestine's epithelium, which acts as a "danger signal" without causing intestinal damage. Despite being challenging to study due to its resistance to in vitro cultivation, recent success in establishing an in vitro SFB culturing system has shed light on its life-cycle. Yet, many aspects of SFB remain unexplored, with molecular details yet to be revealed.
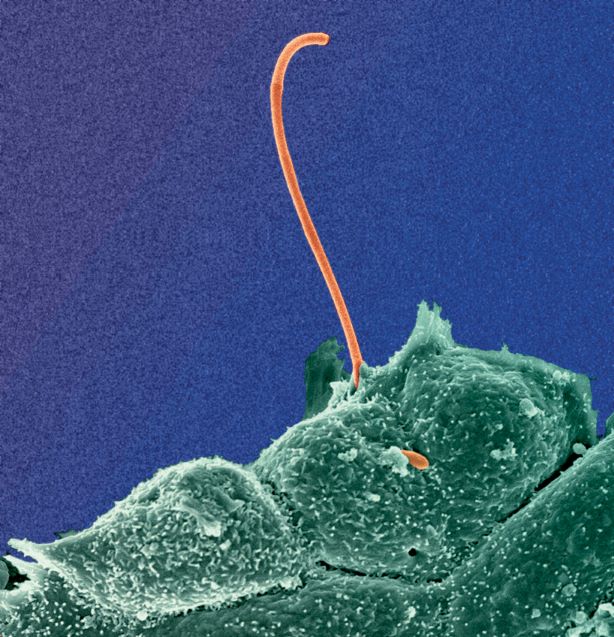
The overall goal of the lab is to obtain a comprehensive view of the SFB life-cycle and how its intimate interaction with the host leads to its potent immunomodulatory effects.
Our main research objectives are therefore to:
- Develop SFB genetic manipulation techniques
- Characterize in detail the SFB life-cycle on a morphological and molecular level
- Analyze the SFB-host interaction on a cellular and molecular level
- Identify host factors involved the SFB-mediated immune activation
- Better understand how niche constraints have shaped SFB genome evolution
- Develop SFB as a novel vaccine delivery system against diarrheal pathogens