Muscle Stem Cells-Niche Interactions
We are studying skeletal muscle stem cells with a focus on the mechanisms of maintenance of cellular quiescence, the nature and function of the extracellular matrix niche and the involvement of the Notch signalling pathway.
We combine mouse genetics with high-throughput and single-cell molecular approaches to dissect the signalling axes regulating muscle stem cells (MuSCs) during growth, homeostasis, regeneration and pathological conditions, such as muscle dystrophy. Our work has contributed to the notion of a self-made quiescence niche by the stem cells and identified Notch as an upstream regulator of a dynamic extracellular matrix network. Also, we have developed specialised protocols for the isolation of quiescent MuSCs, which have uncovered a new early activation state of muscle stem cells and generated comprehensive transcriptional maps of the quiescence-to-activation transition.
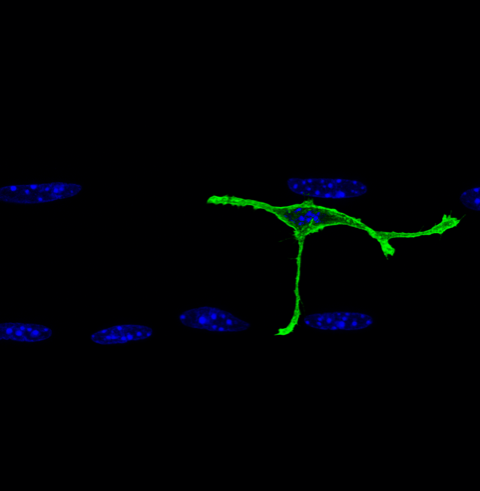
Satellite cell on a myofiber
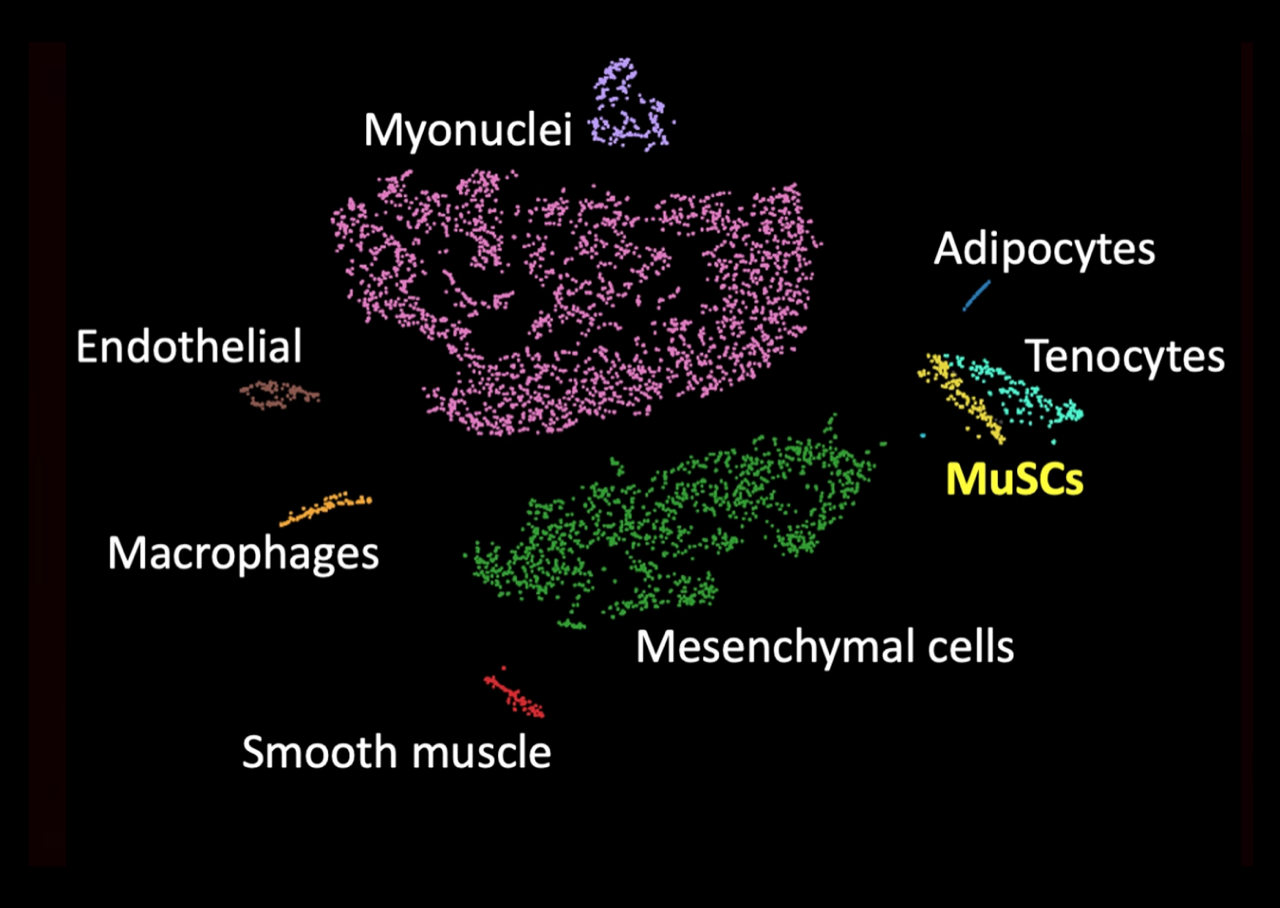
Single-nucleus RNAseq on TA muscle
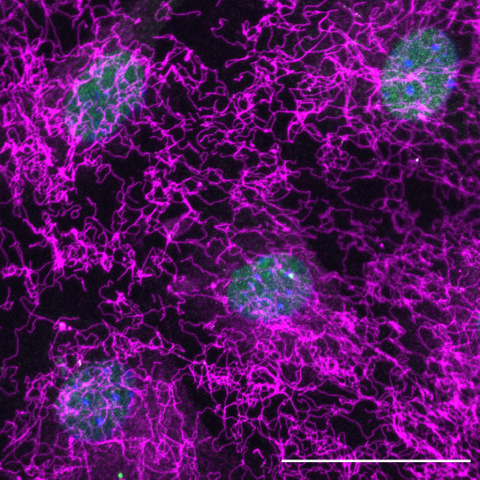
FBN1 expression by NCID myoblasts
The team’s projects are divided into three main axes:
Axis 1. Building the extracellular matrix niche of muscle stem cells - Over ten years ago, we identified Notch signalling as the first quiescence regulator of MuSCs. By combining ChIP-seq with transcriptomic analyses, we have identified new Notch targets that propose novel mechanisms by which this pathway safeguards quiescence. Our working hypothesis is that MuSCs are at the core of an ECM network, which is required for their maintenance in homeostasis and regeneration.
Axis 2. Mechanisms controlling quiescence and activation of muscle stem cells - The capacity of the skeletal muscle to regenerate depends on MuSCs that are G0 arrested in healthy muscles. During our ongoing efforts to understand the role of the quiescent niche and the mechanisms of stem cell activation, we realised that standard cell dissociation methods induce rapid transcriptional and epigenetic changes of unexpected magnitude. By developing specialised protocols to preserve the molecular profile of cells, we have identified candidate gene circuits that operate in quiescent and activated MuSCs.
Axis 3. Investigate the origin and function of FAPs - In addition to MuSCs, fibro-adipogenic progenitors (FAPs) in the muscle are essential for tissue homeostasis and regeneration. FAPs are mesenchymal fibroblastic cells located in the interstitial space between muscle fibers. In pathological contexts, such as the Duchenne muscular dystrophy, FAPs aberrantly proliferate and differentiate, leading to increased fibrosis and fat infiltrations. With a focus on Notch, we aim to identify the pathways that regulate the generation and maintenance of FAPs, as well as what in dysregulated in pathological conditions leading to the establishment of fibrosis.










